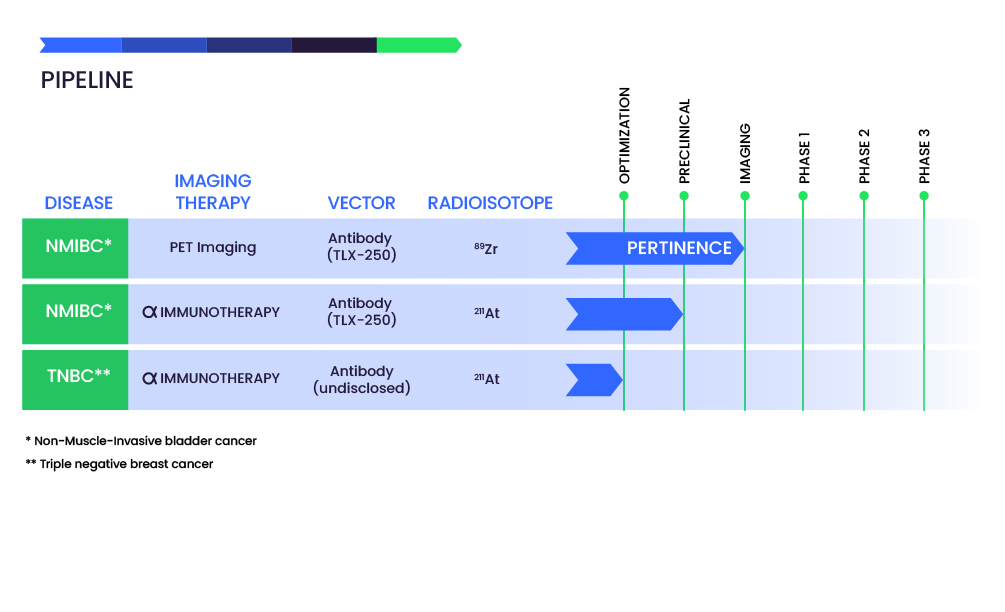
Pipeline
The radiopharmaceuticals developed by Atonco meet the two characteristics of astatine-211, namely a relatively short half-life (7.2 h) requiring a rapidly accessible tumor target and a very short alpha particle path length requiring a thin tumor target.

Bladder Cancer
Alpha-immunotherapy of non-muscle-invasive bladder cancer (NMIC) with an astatine-211-labeled chimeric anti-CAIX antibody, girentuximab.
Our first development targets non-muscle invasive bladder cancer refractory to BCG and chemotherapy and awaiting radical cystectomy.
The radiopharmaceutical candidate is an anti-CAIX antibody labeled with astatine-211 using a proprietary radiolabeling technology, developed in Nantes, France.
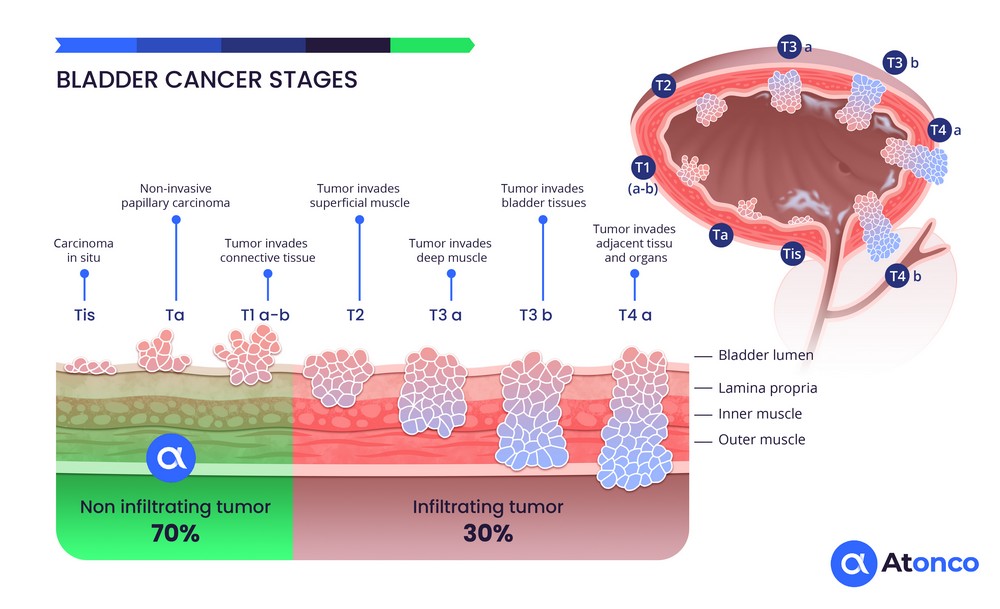
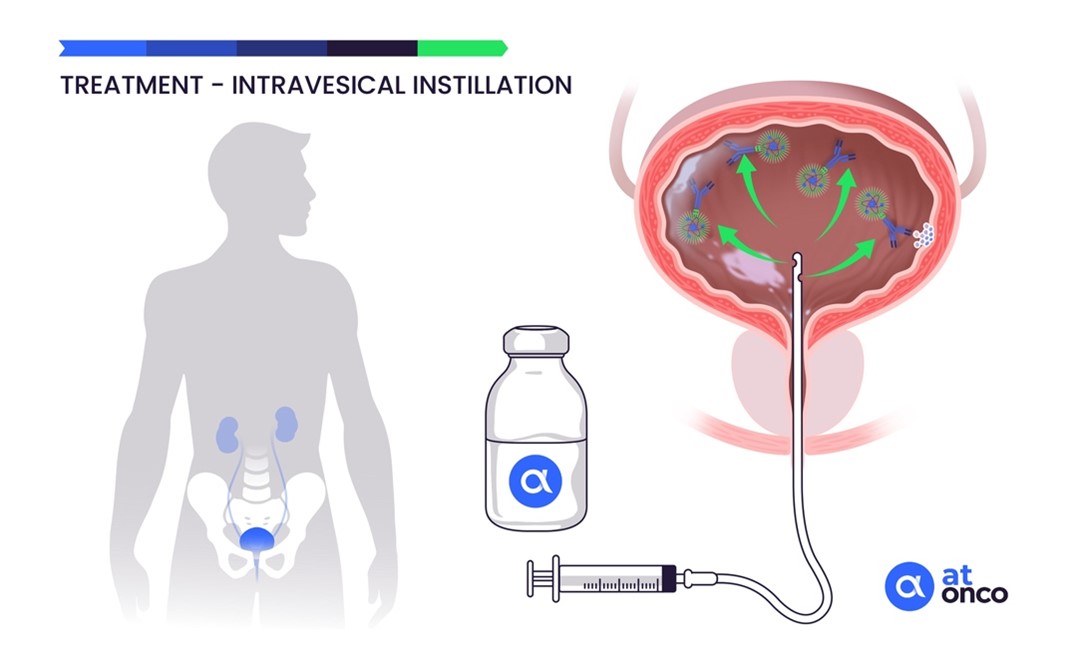
The CAIX antigen is expressed on the membrane of cancer cells in superficial, non-muscle-invasive bladder tumors and therefore directly and rapidly accessible to the radiolabeled anti-CAIX antibody instilled intravesically. In addition, recurrences that frequently occur after repeated BCG or chemotherapy treatments consist of thin layers of cancer cells that respond well to the very short path length of the emitted alpha particles. Thus, the situation of non-muscle-invasive bladder cancer corresponds well to the radiophysical characteristics of alpha particles.
In addition, the clinical situation of patients refractory to conventional treatment using BCG therapy or chemotherapy and for whom the risk of an uncomfortable radical cystectomy justifies the implementation of the innovative alpha immunotherapy delivered by intravesical instillation.
Bibliography
• Pfost B, Seidl C, Autenrieth M, et al. Intravesical a-radioimmunotherapy with 213Bi-anti-EGFR-mAb defeats human bladder carcinoma in xenografted nude mice. J Nucl Med 2009;50:1700-1708
• Autenrieth ME, Seidl C, Bruchertseifer F, et al. Treatment of carcinoma in situ of the urinary bladder with an alpha-emitter immunoconjugate targeting the epidermal growth factor receptor: a pilot study. Eur J Nucl Med Mol Imaging 2018;45:1364-1371.
